Plant parts charge supercapacitors' step forward
 Trees may soon play a role in making high-tech energy storage devices, after an exciting chemical discovery.
Trees may soon play a role in making high-tech energy storage devices, after an exciting chemical discovery.
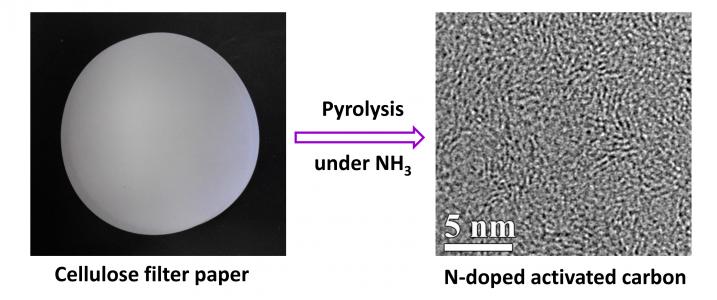
Researchers have found that cellulose – the most abundant organic polymer on Earth – can be heated in a furnace in the presence of ammonia, and turned into the building blocks for supercapacitors.
Cellulose – the key component of virtually all plant life – may soon be used to build the electrodes for incredibly high-powered energy devices.
Supercapacitors are extraordinarily intense energy materials with a wide range of industrial applications. As batteries they are capable of storing and discharging massive amounts of energy, almost instantaneously.
Their widespread use has been hindered by the difficulty of producing high-quality carbon electrodes, as well as related costs.
The new approach developed at Oregon State University in the US can produce nitrogen-doped, nanoporous carbon membranes – the electrodes of a supercapacitor – at low cost, quickly, in an environmentally benign process.
The only byproduct is methane, which could be used immediately as a fuel or for other purposes.
“For the first time we’ve proven that you can react cellulose with ammonia and create these N-doped nanoporous carbon membranes,” assistant professor of chemistry Xiulei Ji said.
“It’s surprising that such a basic reaction was not reported before.”
“Not only are there industrial applications, but this opens a whole new scientific area, studying reducing gas agents for carbon activation.”
Supercapacitors use extraordinarily thin carbon membranes - a single gram of which has a surface area of nearly 2,000 square metres.
The new process can create them in a single-step reaction that is fast and inexpensive.
The process begins with simple cellulose filter paper, which is then exposed to high heat and ammonia.
This converts the cellulose to a nanoporous carbon material needed for supercapacitors, and should enable them to be produced, en masse, much cheaper than before.
“There are many applications of supercapacitors around the world, but right now the field is constrained by cost,” Ji said.
“If we use this very fast, simple process to make these devices much less expensive, there could be huge benefits.”
The study has been published in NanoLetters.








 Print
Print