Better silver treatment for drugs that miss
 Medical engineers have created a new particle-based drug delivery system with some key advantages over previous versions.
Medical engineers have created a new particle-based drug delivery system with some key advantages over previous versions.
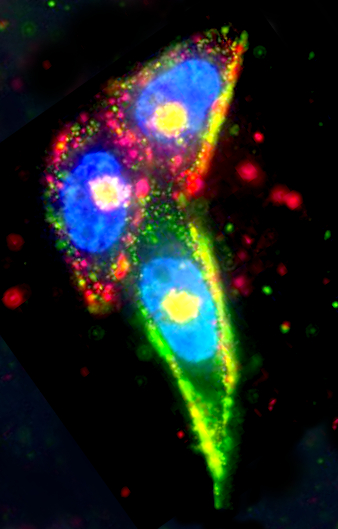
The device is a small, spherical, silver nanoparticle, which is encased in a shell coated with a peptide that enables it to target tumour cells.
The most intriguing part of the design is the fact that the shell is etchable.
The etching means that nanoparticles which do not hit their target are broken down and eliminated by the body’s natural functions.
It could lead to a new way of delivering drugs for virtually any purpose, with a reduced risk of damaging the wrong target, and which is easier to monitor after it is injected.
The core of the nanoparticle employs a phenomenon called plasmonics.
In plasmonics, metals such as gold and silver resonate in light and concentrate the electromagnetic field near the surface.
This means that normal fluorescent dyes are enhanced, appearing about ten times brighter than their natural state when no metal is present.
But when the core is etched, the enhancement goes away and the particle becomes dim.
Using the latest version of the nano-etching techniques, a team at the University of California have refined the method to allow the body’s chemicals to rapidly disassemble and remove the silver nanoparticles outside living cells.
It leaves only the intact nanoparticles for imaging or quantification, revealing which cells have been targeted and how much each cell took in.
This method for removing nanoparticles unable to penetrate target cells is unique.
“By focusing on the nanoparticles that actually got into cells... we can then understand which cells were targeted and study the tissue transport pathways in more detail,” said researcher Gary Braun.
Some drugs are able to pass through the cell membrane on their own, but many drugs, especially RNA and DNA genetic drugs, are charged molecules that are blocked by the membrane. These drugs must be taken in through endocytosis, the process by which cells absorb molecules by engulfing them.
“This typically requires a nanoparticle carrier to protect the drug and carry it into the cell,” Braun said.
“And that's what we measured; the internalization of a carrier via endocytosis.”
Researchers are able to vary the exterior coating of the silver nanoparticles, to compare the efficiency of tumour targeting and internalisation.
Switching out the surface agent enables the targeting of different diseases — or organisms in the case of bacteria — through the use of different target receptors.
“They also have potential applications in combating infections. Dangerous infections caused by bacteria that are resistant to all antibiotics are getting more common, and new approaches to deal with this problem are desperately needed. Silver is a locally used antibacterial agent and our targeting technology may make it possible to use silver nanoparticles in treating infections anywhere in the body,” said fellow researcher Erkki Ruoslaht.
The study has been published in Nature Materials.








 Print
Print